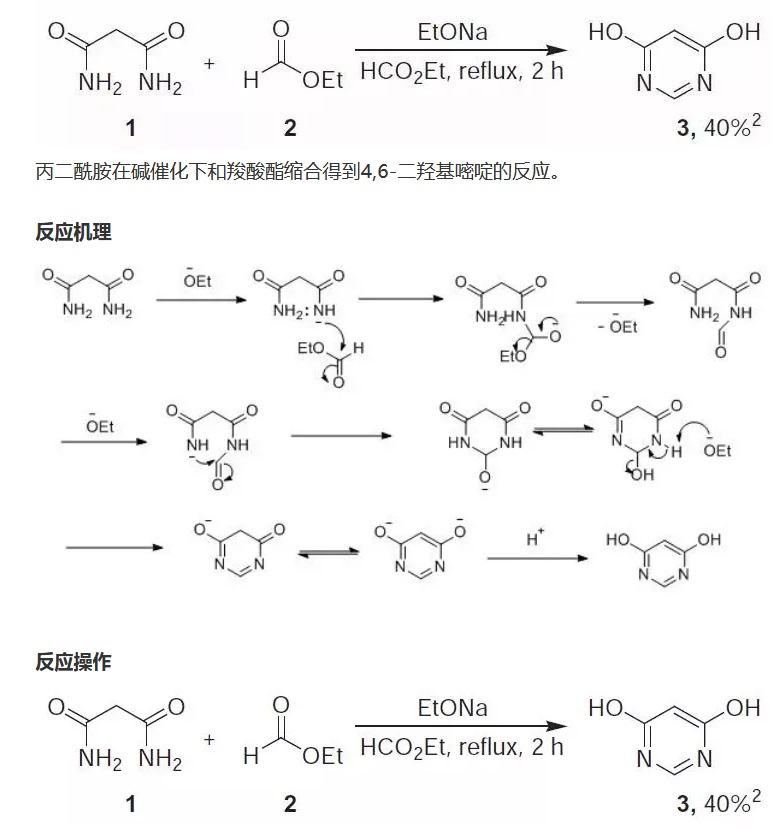
4,6-Dihydroxypyrimidine (3). To NaOEt (from 4.6 g, 0.2 mol Na in 150mL EtOH) was added 1 (10.2 g, 0.1 mol), followed by ethyl formate 2 (11 g, 0.14 mol). The mixture was refluxed for 2 h and after 24 h at 20 C, the crystalline product was filtered and washed with EtOH. The product was dissolved
in water (50 mL) and acidified with 5N HCl. Filtration gave 4.5 g of 3, HCl (40%), mp> 300 C.
【Hull R, J Chem Soc., 1951, 2214】
相关文献
1 Remfry FGP J Chem Soc 1911 610
2 Hull R J Chem Soc 1951 2214
3 Brown DJ J Chem Soc 1964 3204, 1956, 2312
4 Budesinsky Z Coll Czech Chem Comm 1965 30 3730
5 Fischer RW Org Proc Res Dev 2001 5 581
编译自:Organic Syntheses Based On Name Reactions, 3RdEd, A. Hassner, Page 398.

